|
|
| | |
|
|
| CLINICAL STUDY |
|
| SUMMARY OF CLINICAL STUDY |
|
|
This is a summary report.
BIOGEM has made the Efficacy Test for Hair Volume (increase hair density) blind clinical study on DEC 20, 2013 by an US
FDA- registered Laboratory in California, USA.
|
|
| 1. ABSTRACT |
|
|
Study Title:
Study Number:
Testing Facility:
Study Sponsor:
Test Products:
|
Evaluation of the Efficacy of a Hair Regimen to improve the Appearance of Hair Volume.
B**13-071
B** Clinical Services Division
Phoenix, Az. USA
B** Testing Services, Inc.
Torrence, Ca. USA
BIOGEM
Appolos Inc.
Anaheim, Ca.USA
Biogem Revitalizing Hair Shampoo-Normal to Dry
Biogem Revitalizing Hair Shampoo-Oily
Biogem Revitalizing Hair Conditioner - All Hair Types
Biogem Leave-In Treatment
|
|
|
Safety Prerequisite :
FDA requires testing of products prior to determine its safety. The repeat insult patch test will determine the potential of the test product to
cause allergic retains or skin irritation in human subjects. Human Repeat Insult patch test(HRIPT) 50 Subjects 8-10 weeks: Passed
Non-Irritating, Clinically tested and Allergy tested on Sept 25, 2013. [Appedix A]
Study Duration
12 weeks (Sep 30, 2013- Dec 20, 2013)
Subjects:
1. Number of Subjects: 29 Healthy male and female subjects
2. Age: 18-65
Research Standard
This clinical study was conducted in accordance with the International Conference of Harmonization Tripartite Guideline on Good Clinical
Practice, applicable FDA refulations/guidelines set forth in 21 CFR Parts 11, and 50 and standard practices of B** Testing Services
|
|
| 2. SUMMARY OF RESULTS |
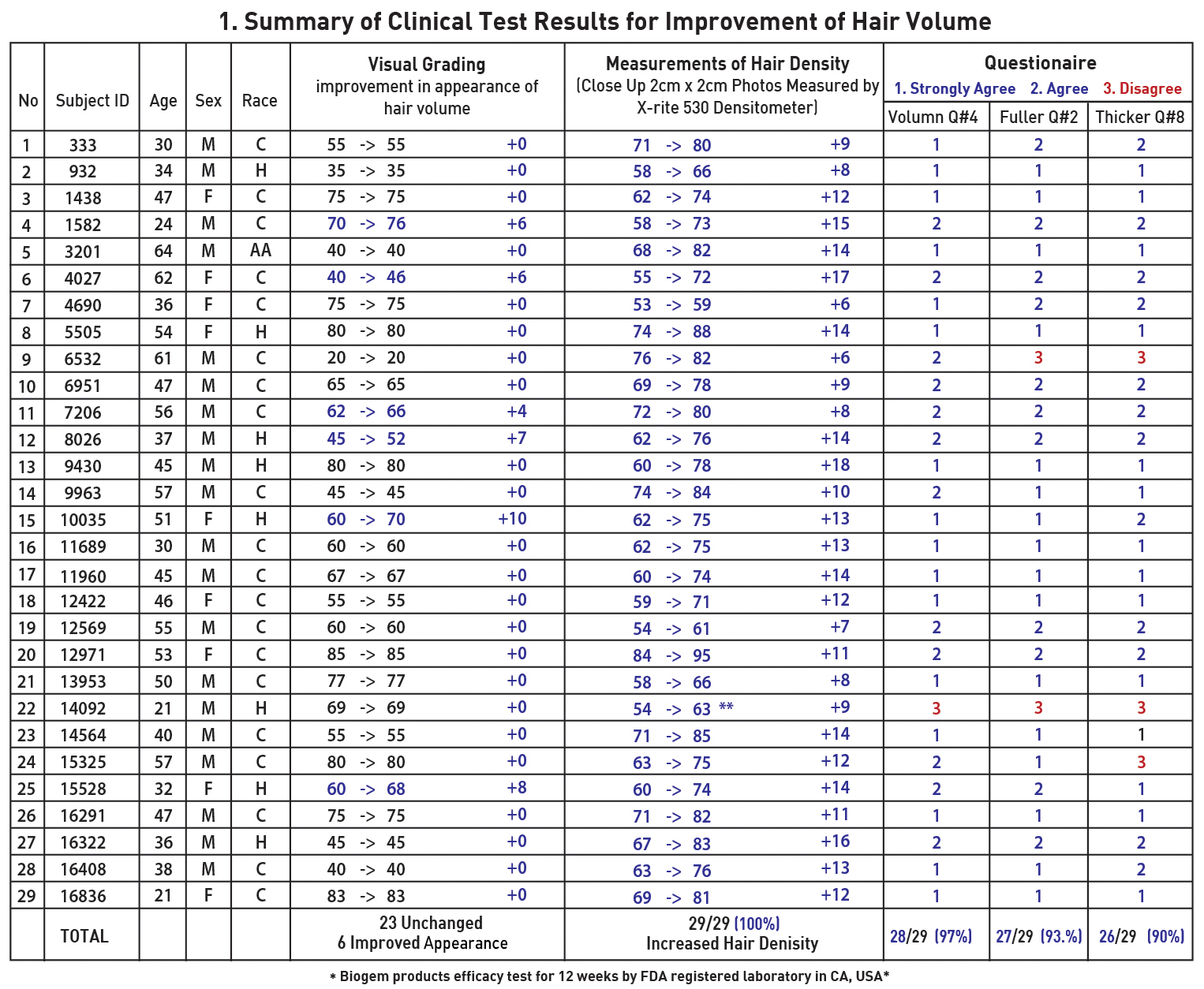 |
|
|
|
|
KEY RATE
1. 100% of subjects : Stopped Balding
2. 021% of subjects : Improved Appearance
3. 100% Improved Hair Density (Close up Photo)
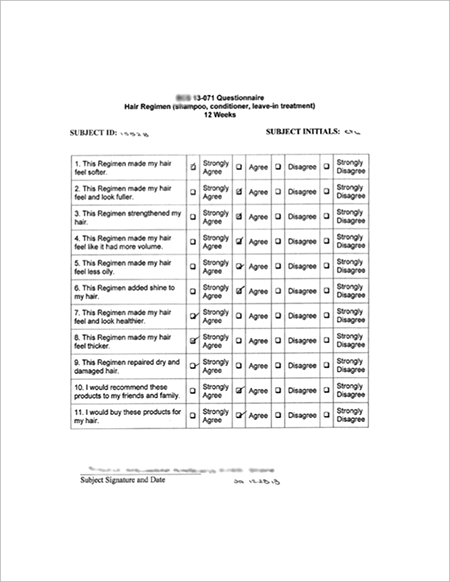
● SUBJECT PERCEPTION RATES (Questionnaire)
1. 100% - More Healthier
2. 97% - More Volume
3. 93% - More Fuller
4. 90% - More Thicker |
● Measuring close-up photo by X-Rite 530 densitometer
(Instrumental Measurement by Densitometer in outsourcing service)
|
|
3. CONCLUSIONS
|
This study strongly demonstrated
1. BIOGEM products are safe: Under the HRIPT (Human Repeat Insult Patch Test 8 weeks 50 Subjects), results show no signs or symptoms of irritation or allergy.
2. BIOGEM products work remarkably well:
a. The stabilization of hair loss (100% of subjects stopped balding) over the study period.
b. Examination of efficacy data based on hair density measurements demonstrated 100% of subjects increased hair density and an overall statistical significant clinical benefit in improving hair volume. Additionally, this study more objectively shows a close match with subjects perception improved rate (97%) of hair volume by subjects post-treatment questionnaire.
This clinical proof is very superior because the results are much faster and better improvement than any other pharmaceutical (drug) hair loss treatments (Example: leading brand using Minoxidil [drug] 1year test result only 30% of subjects cosmetically acceptable improvement).
Although numerous clinical trials in several other countries were conducted, we could not find any other non-pharmaceutical hair treatment has been as thoroughly safety and efficacy clinically tested like BIOGEM uniquely in accordance with USFDA and GCP guidelines and conducted by FDA-registered laboratory in USA.
Therefore, BIOGEM products are your best guarantee that you get all the clinically proven benefits such as more volume, thicker, fuller and healthier hair
(Above comments added by BIOGEM)
|
|
|
4. APPENDIX (EVIDENCE REPORT AND RAW DATA)
|
A. SAFETY TEST
|
| REVITALIZING SHAMPOO (Normal to Dry) |
REVITALIZING SHAMPOO (Oily) |
 |
 |
 |
 |
|
|
| REVITALIZING CONDITIONER |
LEAVE-IN TREATMENT
|
 |
 |
 |
 |
|
|
|
|
|
|
|
|
| B. EFFICACY TEST |
 |
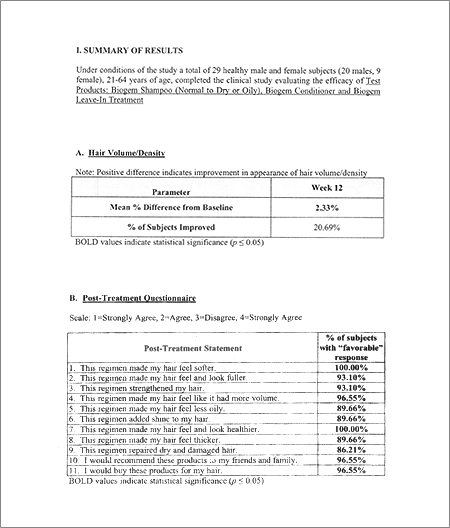 |
|
|
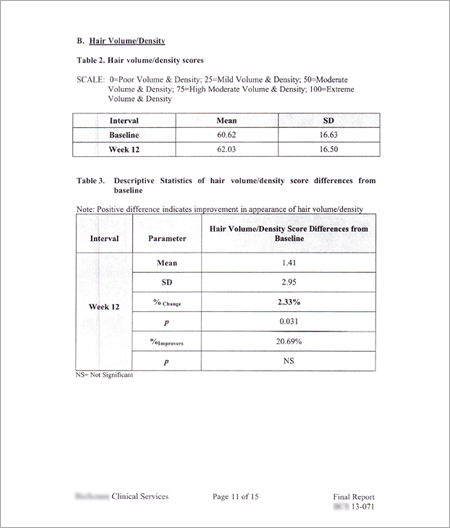 |
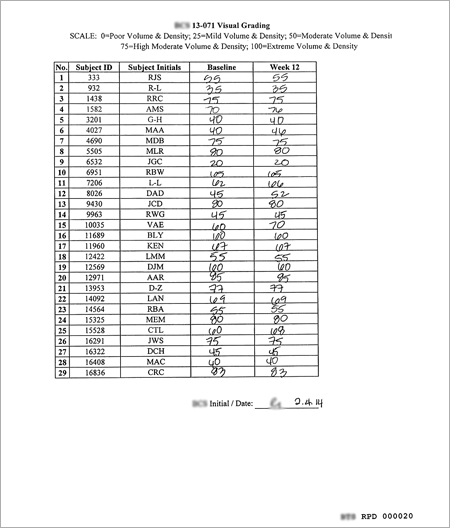 |
|
| ● Sample of Photos with Measurement |
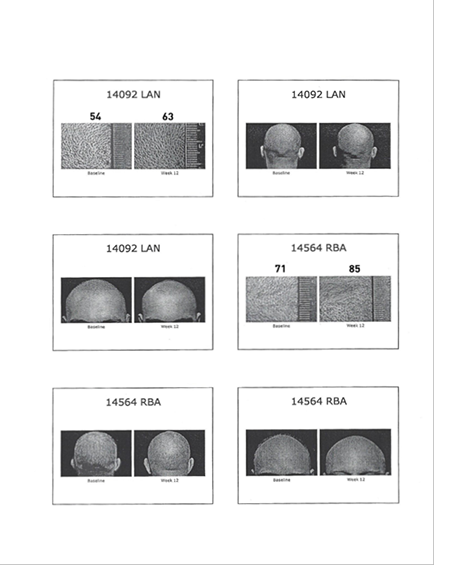 |
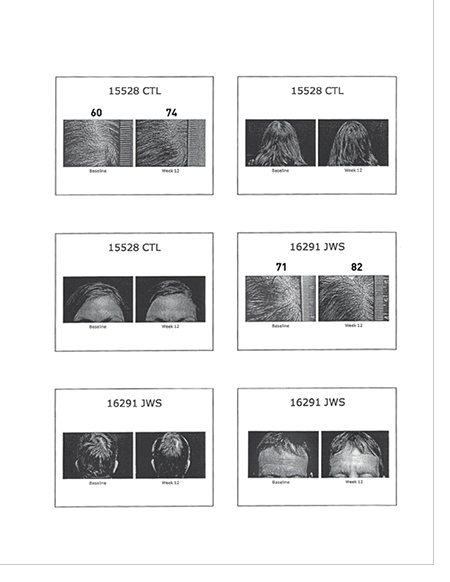 |
|
|
|
|
| ● Sample of Subject Post-Treatment Questionnaire |
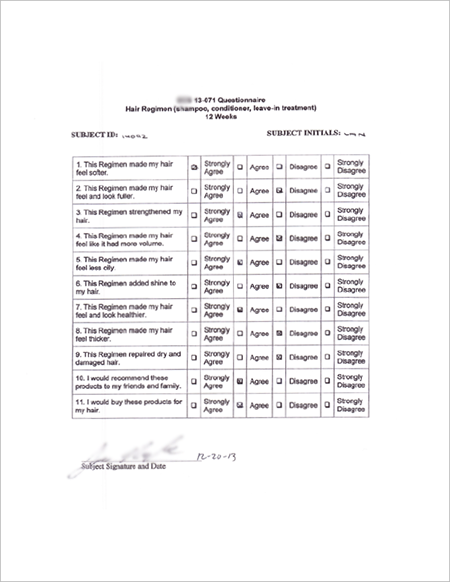 |
 |
|
|
|
|
● References (QVC, HSN Testing Guidlines)
|
 |
|
|
|
|
| |
| | |
|
|